For wound care specifically, an ideal therapy would address concerns such as desiccation (loss of moisture from the wound), long term storage, bacterial infection, preventing debilitating scar formation, and promoting proper skin regeneration (growth of skin appendages, such as hair follicles, and other cutaneous glands) within the wound (Figure 1). Over the past decade, there has been increasing evidence that therapeutic hydrogels may address many of these concerns and promote natural skin regeneration, based on strong and promising laboratory and preclinical research findings. These dressings can be kept lyophilized (dry), making them lightweight, portable, and shelf stable. In the clinic, they can be simply unpackaged and soaked in saline to re-hydrate it, providing coverage across and preventing desiccation of the wound to facilitate healing.
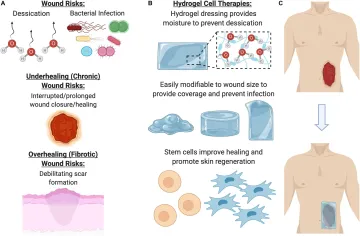
Figure 1. Benefits of using a hydrogel dressing to deliver cellular therapies. (A) Open wounds are at risk for desiccation (loss of moisture) and bacterial infection, as well either underhealing or overhealing. (B) To address these major risk factors, hydrogel dressings provide coverage and moisture, as well as a beneficial ECM environment for cells to grow in. (C) Once the hydrogel has been seeded with cells, it can be laid on the wound to promote healing and regeneration.
Hydrogels have a unique set of properties which make them an ideal candidate for wound dressings. Their high water content confers physical similarity and biocompatibility to body tissues and maintains a moist environment around the wound interface. Hydrogels can be used as a supportive scaffold to deliver therapeutic cells safely to the wound site and shield the delivered cells from immune system attack while retaining permeability to therapeutic, signaling, and metabolic factors. The hydrogel microenvironment can be tightly modified to support cells by adjusting numerous biophysical and biochemical properties, such as hydrogel–cell interactions, cell adhesion, microstructure, and degradability.
Our group has engineered novel pullulan-collagen hydrogels with tunable, soft biomechanical properties and biocompatibility for cell-based therapy encapsulation. To develop a soft, biocompatible hydrogel that recapitulates the three-dimensional organization of the native ECM, we combined pullulan, a linear homopolysaccharide produced by the fungus Aureobasidium pullulans with type I collagen. This material was crosslinked with sodium trimetaphosphate under alkaline conditions, and potassium chloride salt (KCl) was used as a porogen for in-gel crystallization (Wong et al., 2011b). These unique engineered hydrogels provide several key advantages over traditional materials. First, pullulan-collagen hydrogels best approximate the porous ultrastructure of native reticular ECM based on comparison of fiber length and crosslink distance using a network extraction analysis. Moreover, altering the concentration of the collagen:pullulan ratio enables engineering of the mechanical properties such as hydrogel stiffness and effective porosity with relative ease. Additionally, we have conducted both in vitro and in vivo tests to demonstrate the biocompatibility of pullulan-collagen hydrogels.
By using capillary forces, cells can also be optimally seeded into hydrogels at the point of care. First, a cellular suspension is placed on top of a hydrophobic surface (parafilm wax paper); a dry hydrogel is then placed on top of this suspension, resulting in active absorption of cells into the pores of the scaffold through capillary, hydrophobic, and entropic forces (Garg et al., 2014). These hydrogels are able to support the growth of multiple cell types including fibroblasts, endothelial cells, and mesenchymal stromal cells, with minimal cytotoxicity (Figure 2). Use of these hydrogels with a variety of different cells has been shown to improve wound healing across various disease states.
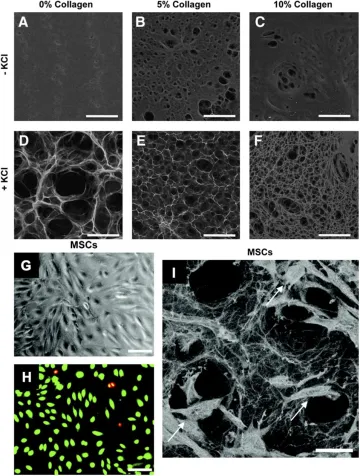
Figure 2. Pullulan-collagen hydrogel can be easily modified across a wide range of factors and provides biocompatibility with stem cells. (A–F)Scanning electron microscopy (SEM) imaging demonstrates that varying collagen and KCl concentrations significantly alters the porosity of the hydrogels. Hydrogels fabricated with KCl showed increased porosity, while increasing collagen concentrations decreased porosity. Scale bar 100 μm. (G,H) Brightfield imaging and fluorescent imaging of live (green) dead (red/yellow) stain shows successful in vitro cellular incorporation of MSCs in the hydrogels. Scale bar 50 μm. (I) SEM shows MSCs (arrows) viably incorporated within the pullulan-collagen hydrogels. Scale bar 25 μm.
Related Publications
Henn D, Chen K, Fehlmann T, Trotsyuk AA, Sivaraj D, Maan ZN, Bonham CA Jr, Barrera JA, Mays CJ, Greco AH, Moortgat Illouz SE, Lin JQ, Steele SR, Foster DS, Padmanabhan J, Momeni A, Nguyen D, Wan DC, Kneser U, Januszyk M, Keller A, Longaker MT, Gurtner GC. Xenogeneic skin transplantation promotes angiogenesis and tissue regeneration through activated Trem2+ macrophages. Sci Adv. 2021 Dec 3;7(49):eabi4528. doi: 10.1126/sciadv.abi4528. Epub 2021 Dec 1. PMID: 34851663; PMCID: PMC8635426.
Wong VW, Rustad KC, Galvez MG, Neofyotou E, Glotzbach JP, Januszyk M, Major MR, Sorkin M, Longaker MT, Rajadas J, Gurtner GC. Engineered Pullulan-Collagen Composite Dermal Hydrogels Improve Early Cutaneous Wound Healing. Tissue Eng Part A. 2011 Mar;17(5-6):631-44. PMID: 20919949
Sivaraj D, Chen K, Chattopadhyay A, Henn D, Wu W, Noishiki C, Magbual NJ, Mittal S, Mermin-Bunnell AM, Bonham CA, Trotsyuk AA, Barrera JA, Padmanabhan J, Januszyk M, Gurtner GC. Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing. Front Bioeng Biotechnol. 2021 May 3;9:660145. doi: 10.3389/fbioe.2021.660145. PMID: 34012956; PMCID: PMC8126987.
Chen K, Sivaraj D, Davitt MF, Leeolou MC, Henn D, Steele SR, Huskins SL, Trotsyuk AA, Kussie HC, Greco AH, Padmanabhan J, Perrault DP, Zamaleeva AI, Longaker MT, Gurtner GC. Pullulan-Collagen hydrogel wound dressing promotes dermal remodelling and wound healing compared to commercially available collagen dressings. Wound Repair Regen. 2022 May;30(3):397-408. doi: 10.1111/wrr.13012. Epub 2022 Apr 18. PMID: 35384131; PMCID: PMC9321852.
Barrera JA, Trotsyuk AA, Maan ZN, Bonham CA, Larson MR, Mittermiller PA, Henn D, Chen K, Mays CJ, Mittal S, Mermin-Bunnell AM, Sivaraj D, Jing S, Rodrigues M, Kwon SH, Noishiki C, Padmanabhan J, Jiang Y, Niu S, Inayathullah M, Rajadas J, Januszyk M, Gurtner GC. Adipose-Derived Stromal Cells Seeded in Pullulan-Collagen Hydrogels Improve Healing in Murine Burns. Tissue Eng Part A. 2021 Jun;27(11-12):844-856. doi: 10.1089/ten.TEA.2020.0320. Epub 2021 May 27. PMID: 33789446.
