An estimated $170 billion is spent annually on biomedical implants that range from lifesaving technologies like pacemakers and implants for reconstructive surgery to biosensors that have revolutionized personalized medicine. All implantable materials used in biomedical devices elicit a host response termed foreign body response (FBR). FBR results in the formation of a collagenous fibrous capsule around the implant, leading to implant failure and rejection and can be effectively corrected only by replacement of the device, necessitating expensive and invasive procedures. For example, 30-50% of pacemakers and 30% of breast implants are replaced due to FBR-related complications. FBR-mediated biomedical implant failure costs the US healthcare economy more than $10 Billion every year (Figure 1).
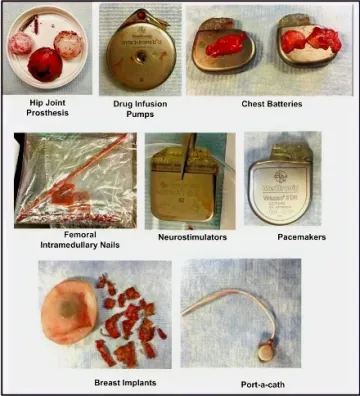
Figure 1. Fibrotic capsules are harvested which surround different biomedical implants as a result of the foreign body response.
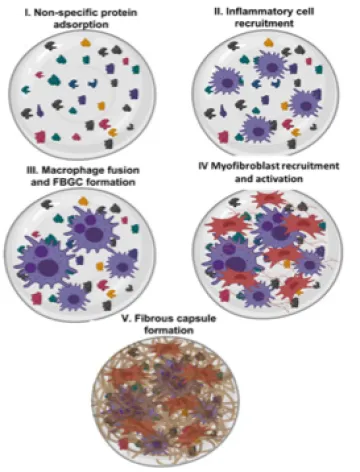
Figure 2. Progression of the FBR to breast implants. The initial wound healing phase of FBR is mediated by inflammatory cells from the circulation. FBR then progresses into fibrosis where local resident cells such as fibroblasts produce a fibrous capsule around the implant.
One key component of fibrosis that is often overlooked in the study of FBR is mechanical signaling. Mechanical cues such as stress, strain and stiffness play an important role in fibrosis such as hypertrophic scarring and lung fibrosis, but its role in FBR has not been examined. The Gurtner laboratory is developing novel small animal models to study the effect of mechanical signaling in FBR to biomedical implants. The Gurtner laboratory is also employing advanced proteomics and single cell sequencing technologies to characterize the heterogeneous population of macrophages and fibroblasts that mediate FBR to biomedical implants. This work will lay the groundwork to develop novel therapeutic strategies to limit FBR and improve biomedical implant function and in vivo lifetimes.
Related Publications:
Sivaraj D, Padmanabhan J, Chen K, Henn D, Noishiki C, Trotsyuk AA, Kussie HC, Leeolou MC, Magbual NJ, Andrikopoulos S, Perrault DP, Barrera JA, Januszyk M, Gurtner GC. IQGAP1-mediated mechanical signaling promotes the foreign body response to biomedical implants. FASEB J. 2022 Feb;36(2):e22007. doi: 10.1096/fj.202101354. PMID: 35051300.
*Read our new paper investigating the importance of mechanical signaling in the foreign body response to biomedical implants
Padmanabhan, J., Chen, K., Sivaraj, D. et al. Allometrically scaling tissue forces drive pathological foreign-body responses to implants via Rac2-activated myeloid cells. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01091-5
Theocharidis G, Veves A. Greater foreign-body responses to big implants. Nat Biomed Eng. 2023 Nov;7(11):1340-1342. doi: 10.1038/s41551-023-01118-x. PMID: 37923821.
