Diabetes mellitus is a major public health problem, costing more than $200 billion and accounting for more than 20% of total health care dollars spent in the United States (US) annually. Clinically, diabetes is associated with microvascular and macrovascular complications as well as global impairment in the neovascular response to tissue hypoxia. Non-healing wounds, resulting from impaired blood vessel formation, are a major cause of diabetes-associated disability. Diabetes-induced impairments in new blood vessel growth greatly increase the severity of the sequelae caused by various ischemic processes, including coronary artery disease, cerebrovascular disease, peripheral vascular disease and impaired wound healing.
Ischemic neovascularization occurs via two fundamentally different pathways: the sprouting of new blood vessels from existing vessels (angiogenesis), and the recruitment, proliferation, and assembly of bone marrow-derived progenitor cells into new vessels (vasculogenesis).
Our laboratory explores the mechanisms by which diabetes impairs neovascularization and wound healing. We have shown that circulating progenitor cells play a role in ischemic tissue recovery and that diabetes impairs new blood vessel formation in response to hypoxia by causing abnormal ischemia-induced signaling as well as impaired progenitor cell proliferation, function, and trafficking. We have found that hyperglycemia hinders the activation of the transcriptional regulator hypoxia-inducible factor 1 alpha (HIF-1a). HIF-1a is normally stabilized under low oxygen tension, allowing it to enter the nucleus and stimulate expression of several downstream signaling molecules including vascular endothelial growth factor (VEGF) and stromal cell-derived factor 1 (SDF-1). These downstream effectors play key roles in normal wound healing, and the exogenous delivery of each has been shown to have beneficial effects in diabetic wounds.
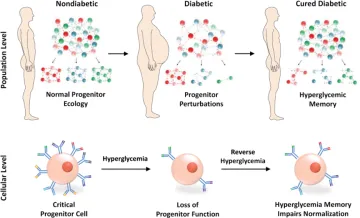
Figure Legend:
Diabetes causes population-wide and single-cell alterations within progenitor cells. Exposure to sustained hyperglycemia causes depletion of critical progenitor cell subsets and alterations within individual progenitor cells. Hyperglycemic memory prevents the normalization of progenitor function on curing of diabetes.
