Cutaneous wound healing following injury is a complex biological process involving the orchestration of the immune system, inflammatory pathways, mechanotransduction pathways, and various types of participating cells. In adult humans, the wound repair process results in the replacement of the damaged functional tissue with a patch of cells (i.e., mostly fibroblasts) and disorganized collagen-rich extracellular matrix that is commonly defined as a scar. While scarring occurs in almost all tissues, it is most apparent in the skin. Cutaneous scarring poses a significant psychological and physiological burden on patients, and an estimated $12 billion are spent annually on scar treatments in the U.S. alone. Keloidal scars, which are characterized by excessive fibrosis in areas of the skin with acne or other minor injuries, represent another major fibrotic skin disorder. Several other skin fibrotic diseases such as Dupuytren’s disease, psoriasis, and systemic sclerosis lead to cutaneous scarring.
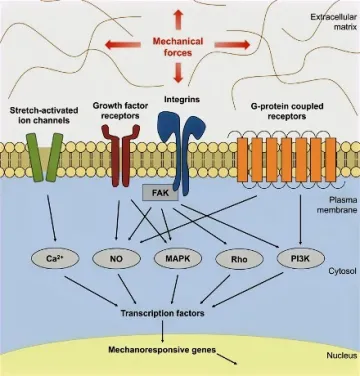
Figure 1. FAK-mediated mechanotransduction. Mechanical forces activate FAK, which activates several downstream effectors and transcriptional factors that mediate cellular mechanotransduction.
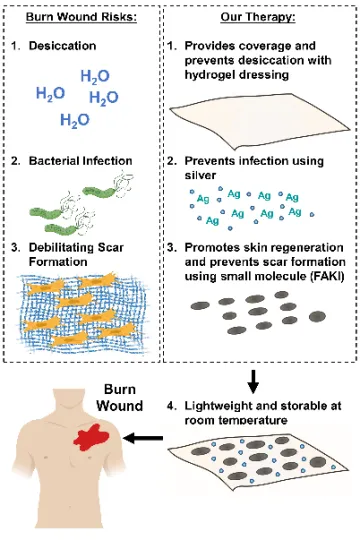
Figure 2. Our therapy addresses major risk factors during wound healing.
Recent progress in the identification of key signaling pathways involved in fibrosis and scar formation has led to the emergence of new therapeutic agents for scar treatment, with promising results in animal studies. Over the past 15 years, our laboratory has demonstrated that activation of mechanotransduction pathways underlie fibrotic wound healing and result in exuberant fibro-proliferation and hypertrophic scar (HTS) formation. In preclinical studies, we have shown that inhibition of the focal adhesion kinase (FAK) pathway significantly attenuates fibrotic HTS formation while accelerating healing of deep dermal injury. To optimally deliver small molecule inhibitors to cutaneous wounds, our laboratory has developed a therapeutic hydrogel dressing that releases FAK inhibitor (FAK-I, VS-6062) in a controlled manner upon contact with open wounds. Large animal studies prove that FAK-I therapy promotes skin regeneration with reduced scar formation and skin appendage reappearance and is safe for topical use in animal models.
Our laboratory is also interested in identifying fibrosis pathways in other organ systems such as the lung, liver, and systemic sclerosis, and devise therapeutic strategies to mitigate fibrotic diseases in multiple organs.
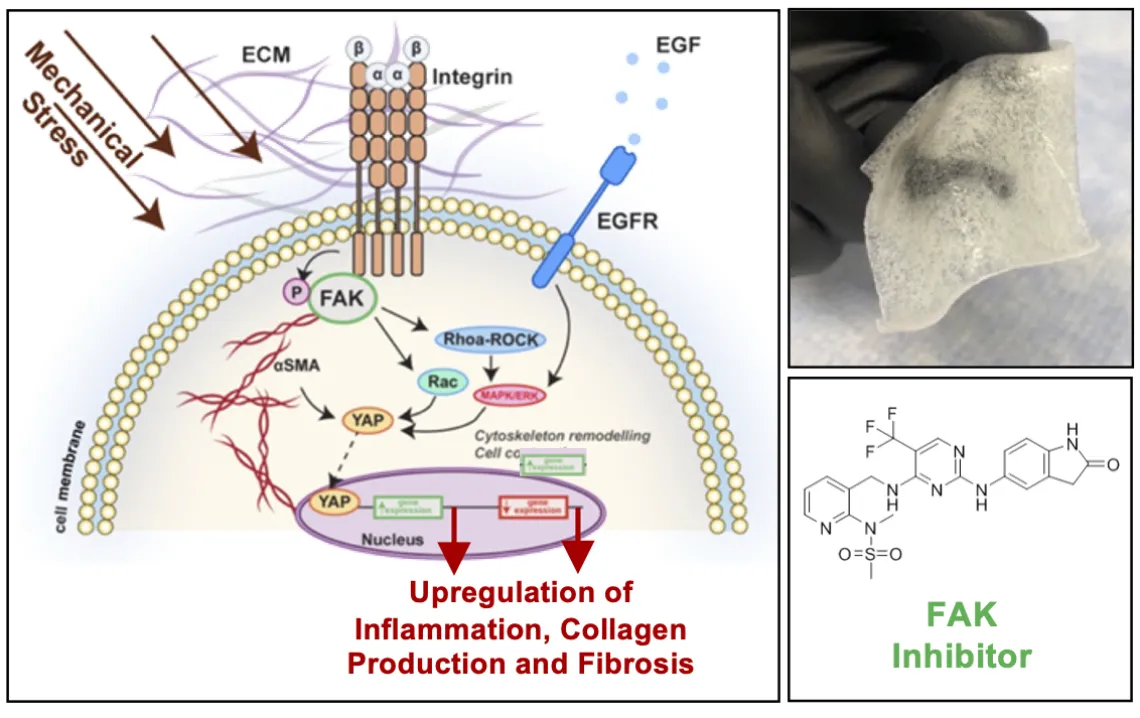
Figure 3: Targeting FAK signaling in a hydrogel formulation can reduce scar formation and promote skin regeneration.
Related publications:
Gurtner GC, Dauskardt RH, Wong VW, Bhatt KA, Wu K, Vial IN, Padois K, Korman JM, Longaker MT. Improving Cutaneous Scar Formation by Controlling the Mechanical Environment: Large Animal and Phase I Studies. Ann Surg. 2011 Aug;254(2):217-25. PMID: 21606834.
Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN,Kuang AA, Longaker MT, Gurtner GC. Focal Adhesion Kinase Links Mechanical Force to Skin Fibrosis via Inflammatory Signaling. Nat Med. 2011 Dec 11;18(1):148-52. PMID: 22157678.
Chen K, Kwon SH, Henn D, Kuehlmann BA, Tevlin R, Bonham CA, Griffin M, Trotsyuk AA, Borrelli MR, Noishiki C, Padmanabhan J, Barrera JA, Maan ZN, Dohi T, Mays CJ, Greco AH, Sivaraj D, Lin JQ, Fehlmann T, Mermin-Bunnell AM, Mittal S, Hu MS, Zamaleeva AI, Keller A, Rajadas J, Longaker MT, Januszyk M, Gurtner GC. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat Commun. 2021 Sep 6;12(1):5256. doi: 10.1038/s41467-021-25410-z. PMID: 34489407; PMCID: PMC8421385.
Chen K, Henn D, Januszyk M, Barrera JA, Noishiki C, Bonham CA, Griffin M, Tevlin R, Carlomagno T, Shannon T, Fehlmann T, Trotsyuk AA, Padmanabhan J, Sivaraj D, Perrault DP, Zamaleeva AI, Mays CJ, Greco AH, Kwon SH, Leeolou MC, Huskins SL, Steele SR, Fischer KS, Kussie HC, Mittal S, Mermin-Bunnell AM, Diaz Deleon NM, Lavin C, Keller A, Longaker MT, Gurtner GC. Disrupting mechanotransduction decreases fibrosis and contracture in split-thickness skin grafting. Sci Transl Med. 2022 May 18;14(645):eabj9152. doi: 10.1126/scitranslmed.abj9152. Epub 2022 May 18. PMID: 35584231.
